Chatting Zonexxx
Lame Linkxxx
- Personal Lame Blogxx
- Mrs Alice the UCSI lecturer
- MkLq the dunno how to describe girl
- Maxcy the leng leng lui
- Munnie the Hot and Sexy girl
- Joanne the Hot and Lamy Girl
- Nicole the guai guai and cute leng lui
- Christine the Cute+quiet leng lui
- Tze Ying the Penang Leng lui
- Shao Ni the dunno who is she leng lui
- Tiffany the dai bag po leng lui
- Yen Chew the good girl
- Linn The lazy one
- Kelly the Kepong Leng lui
- may may the 24hours excited girl
- Jason The excellent guy
- Shiida the Crappy one
- Pika the Neither Thin nor fat boy
- Kevin the Clone of Pika
My Post Content Comment
Lame Followerxxx
Blog Archive
Nuffnangxxx
Danger!!
What is electroplating? Electroplating is the deposition of a metallic coating from the element to an object. It is done by putting a negative charge on the object and exposing it to a solution containing a metal salt. The positively charged metal ions in the salt solution are attracted to the object and reduced to metallic form upon it.

A neutron walks into a bar, sits down and asks for a drink. Finishing, the neutron asks "How much?"
The bartender says, "For you, no charge."
[Explanation for non-chemists: a neutron is a particle that has no charge. Proton are those with positive charge, and electrons those with negative charge.]


 Yeap!! The topic is GLASS. What is glass? How it made? What chemical composition inside it? How many types of glass there on the earth? Those answer will be revealed within the post. We use glass to drink beer, red wine, orange juice, and water.
Yeap!! The topic is GLASS. What is glass? How it made? What chemical composition inside it? How many types of glass there on the earth? Those answer will be revealed within the post. We use glass to drink beer, red wine, orange juice, and water.- solid and hard material.
- Fragile and easily breakable into sharp piece.
- Transparent.
- amorphous structure.
- 100% recycable.
- inert material.
- does not rust, corrode, deteriorate, stain.
- application on architecture, laboratory apparatus, optical instrument, and etc.
- Able to become a deadly weapon.
- Sand (SiO2 silica) - It exist as polymer in its pure state.
- Soda Ash ( sodium carbonate Na2CO3) - At 2000°C the sand will melts. Adding soda will lower the melting point of the sand to 1000°C where the sand is more manageable.
- Limestone ( calcium carbonate CaCO3) or dolomite ( MgCO3) - Addition of soda causes the glass to be water-soluble, soft and not durable. Hence, limestone is added to increase the hardness of the glass, chemical durability and providing insolubility of the material.
- Mixing
- Melting
- Forming
- Cooling
- composed of 60%-75% silica, 12%-18% soda and 5%-12% lime.
- has light transmission and often used for flat glass in window.
- smooth and nonporous surface enable the glass bottle to be easily cleaned.
- resistant to chemical attack.
- does not absorb UV light.
- not resistant to high temperature. (will break when in contact with high temperature liquid)
- example : window glass, drinking glass and etc.
- composed of 54%-65% silica, 18%-38% Lead Oxide (PbO), 13%-15% soda or potash(K2) and various oxide.
- moderate amount of lead increase the durability of glass
- High amount of lead lowers the melting point and decrease the hardness giving a soft surface for the glass.
- High refractive index.
- glass with high lead oxide composition is able to used as radiation shielding glass as lead absorb gamma ray and other harmful radiation before the radiation penetrate the glass.
- Mostly used in nuclear industry.
- Unable to withstand high temperature and sudden change of temperature.
- composed of 70%-80% silica, 7%-13% Boric oxide (B2O3), 4%-8% of sodium oxide(Na2O and potassium oxide (K2O) and 2%-7% or aluminium oxide (Al2O3)
- Greater resistance to temperature change and chemical corrosion
- Used in laboratory, chemical processing plant, and etc.
- greater resistant to thermal shock and increase the accuracy in laboratory experiment.
- Alumina - increase chemical resistance and vicosity in lower temperature change.
- Cerium - Absorb the infrared ray.
- Barium oxide - provide high refractive index.
- Fluorine - provide a cloudy and opaque impression.
Food, is the main component needed daily in human life. We eat cooked chicken, fried fish and so much other food that are well cooked. However, the taste of the food is depends on the chef's cooking skill.

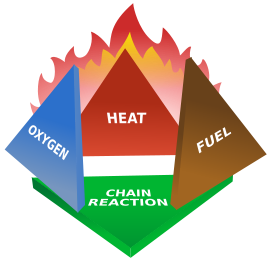
- A rapid chemical reaction.
- Oxidation of combustible matter.
- An exothermic reaction
- Produce reaction product which is carbon dioxide and water.
- Release Heat energy and light energy to surrounding.
- colour of flame vary with the type of impurities in the matter burnt.
- mostly result in conflagration.
- Application of water as the water absorb the heat from the fuel faster than the combustion generate it.
- Application of carbon dioxide to the fire to remove the oxygen to react with the fuel.
- Application of sand or mud to prevent the oxygen to react and absorb the heat.
- Class A- used for common materials such as paper, wood,cardboard or most plastic.
- Class B- for flammable or combustible liquid such as gasoline, paint remover, grease, kerosene or oil.
- Class C- for electrical equipement such as appliances, wiring, circuit breakers and outlets.
- Class D-for combustible metal such as magnesium, titanium, potassium and sodium. Usually found in industry and laboratory.
- BC - regular type of dry chemical extinguisher. Filled with sodium bicarbonate or potassium bicarbonate.
- ABC - multipurpose type of dry chemical extinguisher. Filled with monoammonium phosphate. It is a yellow powder that will leaves sticky residue on the electrical appliances and damage it.
- Pull the pin
- Aim the fire
- Squeece the lever
- Sweep the nozzle

Fart so loud wakes the animals 5 miles away, so stinky kills the animals straight away.
Once upon a time, there was a village called the Threadfin Estuary. There was a big ancient tree which age for 100years. 15 years ago, a couple of bees came to the village.
- hypotension (low blood pressure)
- angioedema ( swelling of lips, neck, face and throat)
- abdominal pain
- anxiety
- itching
- vomiting
- unconsciousness
- fainting
Eight years ago, everything seems new, perfect, nice and attractive. Every cell, every molecules, every organs are new as newly born-ed infant. I love her so much and I slept with her for eight years.
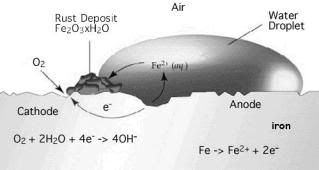
- Alloy Formation = a method where the iron is alloyed with other metals /elements to form alloy which are more resistant to rust
- The barrier (protective layer) method = a method where the iron is prevented from coming into contact with oxygen and water by having the iron surface coated with paint, oil , grease, plastic or other materials. As long as the protective layer is not broken, iron will not rust.
- Electrochemical Method (the use of sacrificial anode) = when iron is in contact with a more electropositive metal, the metal will undergo corrosion, but not the iron
In conclusion, the most effective way for me to prevent the iron from rusting is seeking a professional painting expert to paint my gate since my painting skill is just novice which caused the painting always broken after i painted it for a while.