Eight years ago, everything seems new, perfect, nice and attractive. Every cell, every molecules, every organs are new as newly born-ed infant. I love her so much and I slept with her for eight years.
But now, worse thing come when she is not take care nicely,beautifully and perfectly. I feels very sad because she became ugly, irritating and annoying! I tried a lot of ways to help get her back as last time. But, no matter what i do, i just can't get her back! I miss her a lot! Sometimes, i plan to change to another "her", but eight years of feeling towards her, its hard to let her go. So, i will try whatever it takes to fix her.
Oh ya~! Forget to tell you all, the "she" and "her" that i mentioned just now is my house. U know why i refer my house as female? Because, she can born insect and animals from nowhere! (cold jokes huh?)
The problem my house facing now is RUST! Its came from nowhere and loves to combine with my lovely, pretty steel gate! Now, my gate just like chameleon, change colour from grey to brown. I keep painting it when it get rusty but it only able to cure the symptoms but not the disease.
Before developing a solution, we must find how the disgusting rusting forms. I will assume you all don't know anything about it.
Firstly, do u guys/girls know what atom or molecule made up the iron?
The characteristic of Iron
-a metallic element
-symbol Fe (Ferrum)
-has a atomic number of 26 ( Refer to the periodic table).
-has lustrous and silver in colour of surface.
-exist in many stable isotopes ( Fe-54, Fe-55, Fe-56, Fe-57, Fe-58, Fe-59, Fe-60)
Well, rust is a common term for the corrosion of iron. When the iron rusts, the iron react with oxygen and water to form hydrated iron(III) oxide which is called rust. The number of water molecules associated with the hydrated oxide is not fixed and has a general formula of Fe2O3·nH2O.
The occurence of rusting is actually a electrochemical process which involves the transfer of electrons from iron to oxygen. In order to form rust, some chemical reaction called redox reaction is required. Redox reaction is a combination of oxidation and reduction process.
Oxidation- process of loss of electrons/hydrogen or gain in oxygen or increase in oxidation state by atoms, molecules or ions.
Reduction- process of gain in electrons/hydrogen or loss in oxygen or decrease in oxidation state by atoms, molecules or ions.
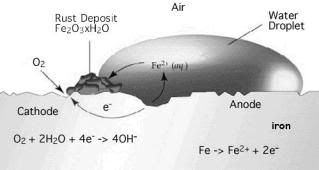
At the middle of the water droplet (where the dissolved oxygen concentration is low) it will act as anode and oxidation of iron occured.
While the region which closer to the outside of the water droplet (where the dissolved oxygen concentration is high) will act as cathode. The electrons donated by the iron is conducted through the iron to the cathode where the oxygen and water is reduced to hydroxide ions.
O2(aq)+ 4e- + 2H2O(l)→ 4OH-(aq)
The iron(II) ions and the hydroxide ions diffuse from their respective poles and get deposited as iron(II) hydroxide.
Fe2+(aq)+ 2OH-(aq)→ Fe(OH)2(s)
The iron(II) hydroxide formed is then oxidised by air to form iron(III) hydroxide which is then converted into rust.
Fe(OH)2(s) + O2(aq) + 2H2O(l) → 4Fe(OH)3(s)
4Fe(OH)3(s) → Fe2O3.nH2O(s) + (3-n)H2O(l)
Now, we already know the rusting process, some solution can be conclude to prevent rusting to occurs. There are several methods that can be used to prevent or maybe, slow down the process of rusting in irons. Those methods are
- Alloy Formation = a method where the iron is alloyed with other metals /elements to form alloy which are more resistant to rust
- The barrier (protective layer) method = a method where the iron is prevented from coming into contact with oxygen and water by having the iron surface coated with paint, oil , grease, plastic or other materials. As long as the protective layer is not broken, iron will not rust.
- Electrochemical Method (the use of sacrificial anode) = when iron is in contact with a more electropositive metal, the metal will undergo corrosion, but not the iron
In conclusion, the most effective way for me to prevent the iron from rusting is seeking a professional painting expert to paint my gate since my painting skill is just novice which caused the painting always broken after i painted it for a while.


0 comments:
Post a Comment